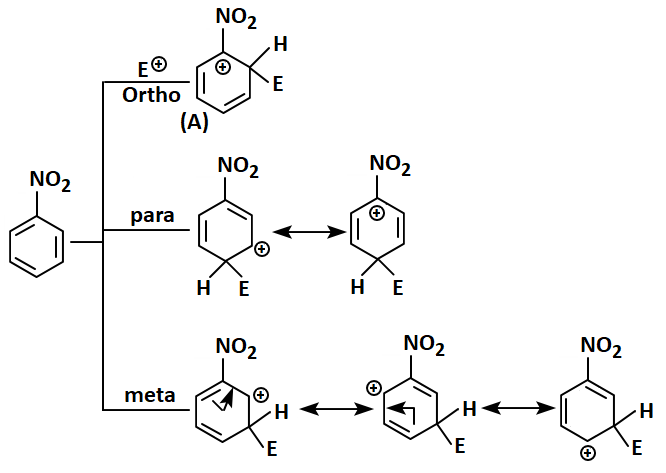
In an electrophilic substitution reaction of nitrobenzene, the nitro group:
| (a) | Deactivates the ring by the inductive effect. |
| (b) | Activates the ring by the inductive effect. |
| (c) | Decreases the charge density at the ortho and para positions of the ring relative to the meta position by resonance. |
| (d) | Increases the charge density at the meta position relative to the ortho and para positions of the ring by resonance. |
1. (c) Only
2. (a) and (c)
3. (b) and (d)
4. (d) only

© 2026 GoodEd Technologies Pvt. Ltd.