Select Chapter Topics:
Consider the following sequence of reactions:
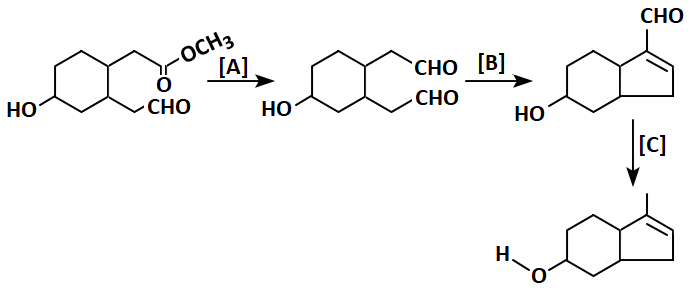
Identify \(\mathrm{A,B,}\) and \(\mathrm{C}\) in the reaction sequence:

Identify \(\mathrm{A,B,}\) and \(\mathrm{C}\) in the reaction sequence:
| 1. | \(\mathrm{A:DiBAL\text-H,}\) \(\mathrm{B:NaOH\text{ (dil)},}\) \(\mathrm{C:Zn\text-Hg/HCl}\) |
| 2. | \(\mathrm{A:LiAlH_4,}\) \(\mathrm{B:KOH\text{ (alcoholic)},}\) \(\mathrm{C:NH_2\text-NH_2/KOH}\) |
| 3. | \(\mathrm{A:DiBAL\text-H,}\) \(\mathrm{B:NaOH\text{ (dil)},}\) \(\mathrm{C:NH_2\text-NH_2/KOH}\) |
| 4. | \(\mathrm{A:NaBH_4,}\) \(\mathrm{B:KOH\text{ (aqueous)},}\) \(\mathrm{C:Zn\text-Hg/HCl}\) |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Consider the given statements:
| Statement I: | The conversion of but-2-ene to ethanal can be obtained by reductive ozonolysis. |
| Statement II: | The conversion of allyl alcohol to propenal is a reduction reaction and can be achieved using the PCC reagent. |
| 1. | Statement I is correct; Statement II is correct. |
| 2. | Statement I is correct; Statement II is incorrect. |
| 3. | Statement I is incorrect; Statement II is correct. |
| 4. | Statement I is incorrect; Statement II is incorrect. |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
Hints
The final product 'A' in the following reaction sequence is-


| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The compound, among the following that cannot give a positive Tollen's test is:
| 1. |  |
2. |  |
| 3. | 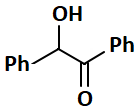 |
4. |  |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
The major product of the following reaction is:


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
NEET - 2025
Please attempt this question first.
Hints
Please attempt this question first.
Identify compound A that starts the following reaction sequence:


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
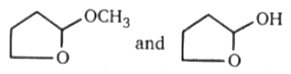
Above compounds can be differentiated by following reagent:
1. 2-4 DNP (Brady reagent)
2. Tollen's reagent
3. Bromine water reagent
4. NaHSO3
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
Hints
Consider the Cannizzaro reaction given below:

The slowest step in this reaction is:
1. attack of \(^-OH\) at the carbonyl group.
2. transfer of hydride to the carbonyl group.
3. abstraction of a proton from the carboxylic acid.
4. deprotonation of \(PhCH_2OH.\)

The slowest step in this reaction is:
1. attack of \(^-OH\) at the carbonyl group.
2. transfer of hydride to the carbonyl group.
3. abstraction of a proton from the carboxylic acid.
4. deprotonation of \(PhCH_2OH.\)
Subtopic: Aldehydes & Ketones: Preparation & Properties |
51%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Which, of the following compounds, can give iodoform reaction?
| 1. | \(~~~~~~~~~~~~O\\ ~~~~~~~~~~~~||\\ HO-C-CH_3\) |
| 2. | \(~~~~~~~~~~~~~O\\ ~~~~~~~~~~~~~~||\\ H_2N-C-CH_3\) |
| 3. | \(~~~~~~~~~~~~~O\\ ~~~~~~~~~~~~~||\\ CH_3 -C - CH_3\) |
| 4. | All of the above |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Given below are two statements:
| Assertion(A): | All aldehydes do not take part in aldol condensation. |
| Reason(R): | In aldol condensation, carbanion is generated by the abstraction of \(\alpha -H\) atom by base. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True and (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Subtopic: Aldehydes & Ketones: Preparation & Properties |
Level 4: Below 35%
Please attempt this question first.
Hints
Please attempt this question first.






