An reaction at an asymmetric carbon of a compound always gives
1. An enantiomer of the substrate
2. A product with opposite optical rotation
3. A mixture of diastereomers
4. A maximum racemized product
The most reactive compound for SN2 reaction among the following is:
| 1. | 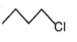 |
2. | 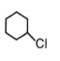 |
| 3. | 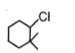 |
4. | 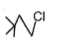 |
| Assertion (A): | The reactivity of alkyl halide for nucleophilic substitution reaction follows the order Rl > RBr > RCl |
| Reason (R): | Leaving ability of halide ions follows the order: I- > Br- > Cl- |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Which of the following compounds undergoes nucleophilic substitution reaction most easily?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The most suitable reagent for the following conversion is:
\(\begin{align} CH_3CH_2CH_2CH_3 \rightarrow\ & CH_3CH_2CH_2CH_2Cl\ +\ \\ & CH_3CH_2CHClCH_3\end{align} \)
1. Cl2/UV light
2. NaCl + H2SO4
3. Cl2 gas in the dark
4. Cl2 gas in the presence of iron in the dark
Compound(s) among the following with an asterisk (*) asymmetric carbon is/are:

1. (a), (b), (c), (d)
2. (a), (b), (c)
3. (b), (c), (d)
4. (a), (c), (d)
Product ‘A’ in the following reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Arrange the compounds in increasing order of rate of reaction towards nucleophilic substitution.

1. (i) < (ii) < (iii)
2. (ii) < (i) < (iii)
3. (iii) < (ii) < (i)
4. (i) < (iii) < (ii)
Arrange the following halides in the decreasing order of SN1 reactivity:
(I)
(II)
(III)
1. I > II > III
2. II > I > III
3. II > III > I
4. III > II > I
Identify (Z) in the following reaction sequence:
C2H5I (X) (Y) (Z):
1. CH3-CH2-CN
2.
3.
4.







