Which of the following statement(s) is/are correct about the elimination reaction of 2-Bromopentane to form pent-2-ene:
(a) -Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
1.
(a), (c), (d)
2.
(b), (c), (d)
3.
(a), (b), (d)
4.
(a), (b), (c)
(a) -Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
An alkene on ozonolysis gives methanal as one of the products. Its structure is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The major product (P) in the following reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
Mark the correct order of reactivity towards the electrophilic aromatic substitution reaction.
| 1. | I > II > III > IV | 2. | IV > III > II > I |
| 3. | II > I > IV > III | 4. | II > I > III > IV |
The major product (P), in the reaction given below is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
Compound x and y respectively in the above reaction is:
| 1. | 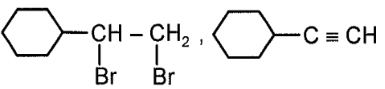 |
| 2. | 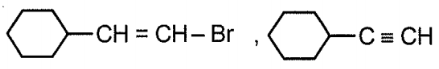 |
| 3. | 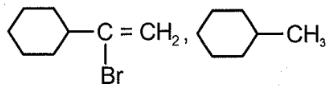 |
| 4. | 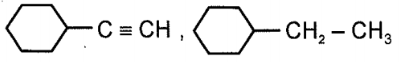 |
Which one of the following compounds of the formula does not decolorize bromine water?
1. But-1-ene
2. But-2-ene
3. Cyclobutane
4. 2-methyl propene
The products formed after ozonolysis of Pent-2-ene are:
| 1. | Ethanal and Methanal | 2. | Ethanal and Propanal |
| 3. | Ethanal and Butanal | 4. | Ethanal and Ethanol |
Decreasing order of acidic behavior of benzene, n-hexane, and ethyne is:
1. Hexane > Ethyne > Benzene
2. Benzene > Hexane > Ethyne
3. Ethyne > Benzene > Hexane
4. Benzene > Ethyne > Hexene
The correct order of decreasing reactivity of the following compounds:
Chlorobenzene, 2,4-Dinitrochlorobenzene, and p-Nitrochlorobenzene
with an electrophile (E+) is :
| 1. | Chlorobenzene > p–Nitrochlorobenzene > 2,4-Dinitrochlorobenzene |
| 2. | p – Nitro chlorobenzene > 2, 4-Dinitrochlorobenzene > Chlorobenzene |
| 3. | Chlorobenzene > 2, 4-Dinitrochlorobenzene > p-Nitrochlorobenzene |
| 4. | 2, 4-Dinitrochlorobenzene > Chlorobenzene > p-Nitrochlorobenzene |








