Select Question Set:
Which of the following methods is incorrect for the synthesis of alkenes?
1. Treatment of alkynes with Na in liquid NH3
2. Heating alkyl halides with alcoholic KOH
3. Treating alkyl halides in aqueous KOH solution
4. Treating vicinal dihalides with Zn metal
Subtopic: Chemical Properties |
78%
Level 2: 60%+
NEET - 2022
Hints
The incorrect reaction among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Chemical Properties |
79%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Match the organic compound given in Column I with their names in Column II. Select the correct option.
1. a=ii; b=iii; c=i; d=iv
2. a=iv; b=i; c=ii; d=iii
3. a=iv; b=iii; c=i; d=ii
4. a=ii; b=iv; c=i; d=iii
| Column I | Column II | |
| (a) CH2Cl2 | (i) | neo-Pentyl chloride |
| (b) (CH3)3CCH2Cl | (ii) | Ethylidene chloride |
| (c) CH3CHCl2 | (iii) | Ethylene dichloride |
(d)  |
(iv) | Methylene chloride |
2. a=iv; b=i; c=ii; d=iii
3. a=iv; b=iii; c=i; d=ii
4. a=ii; b=iv; c=i; d=iii
Subtopic: Introduction and Physical Properties |
91%
Level 1: 80%+
Please attempt this question first.
Hints
Please attempt this question first.
Predict the order of reactivity of the following four isomers towards SN2 reaction.
(I) CH3CH2CH2CH2Cl
(II) CH3CH2CH(Cl)CH3
(III) (CH3)2CHCH2Cl
(IV) (CH3)3CCl
(I) CH3CH2CH2CH2Cl
(II) CH3CH2CH(Cl)CH3
(III) (CH3)2CHCH2Cl
(IV) (CH3)3CCl
| 1. | (IV) > (III) > (II) > (I) |
| 2. | (I) > (II) > (III) > (IV) |
| 3. | (I) > (III) > (II) > (IV) |
| 4. | (IV) > (II) > (III) > (I) |
Subtopic: Chemical Properties |
68%
Level 2: 60%+
NEET - 2022
Hints
Match List I with List II:
Choose the correct answer from the options given below:
| List I Reaction |
List II Type of reaction |
A.  |
I. Nucleophilic substitution |
B.  |
II. Saytzeff elimination |
C.  |
III. Electrophilic addition |
D.  |
IV. Electrophilic substitution |
Choose the correct answer from the options given below:
| (A) | (B) | (C) | (D) | |
| !. | (II) | (IV) | (III) | (I) |
| 2. | (IV) | (II) | (III) | (I) |
| 3. | (IV) | (II) | (I) | (III) |
| 4. | (II) | (IV) | (I) | (III) |
Subtopic: Chemical Properties |
83%
Level 1: 80%+
Please attempt this question first.
Hints
Please attempt this question first.
In which of the following option the reaction does not match with their correct product?
| 1. |  |
| 2. |  |
| 3. | 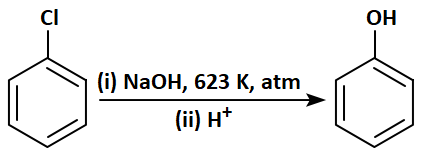 |
| 4. |  |
Subtopic: Chemical Properties |
87%
Level 1: 80%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The product formed in the following reaction sequence is:
| 1. |  |
| 2. | \(CH_3-CH=CH-CH_3 \) |
| 3. | \(CH_3 -CH_2 -CH = CH_2 \) |
| 4. | \(CH_3 -CH_2 -CH_2 -CH_2 -OH \) |
Subtopic: Chemical Properties |
63%
Level 2: 60%+
NEET - 2022
Hints
The correct reaction among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Chemical Properties |
80%
Level 1: 80%+
NEET - 2022
Hints
The number of chiral carbons in 1 molecule of testosterone is:

1. 5
2. 6
3. 7
4. 3

1. 5
2. 6
3. 7
4. 3
Subtopic: Isomerism & Chirality |
63%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Identify the statement that is incorrect regarding chirality:
| 1. | A racemic mixture shows zero optical rotation |
| 2. | SN1 reaction yields a 1:1 mixture of both enantiomers |
| 3. | The product obtained by SN2 reaction of haloalkane having chirality at the reactive site shows the inversion of configuration |
| 4. | Enantiomers are superimposable mirror images on each other |
Subtopic: Chemical Properties |
77%
Level 2: 60%+
NEET - 2022
Hints
Select Question Set:







